AKYNZEO® injection
Discover the clinical data supporting AKYNZEO injection
The efficacy of AKYNZEO injection was established by demonstrating
- Noninferiority of IV palonosetron bolus vs IV Infusion
- Bioequivalence of fosnetupitant to netupitant
Safety of AKYNZEO injection was established based on:
Palonosetron administered as an IV infusion was shown to be noninferior to a bolus IV push1
- Multicenter, multinational, randomized, double-blind study
- 425 adult cancer patients receiving a non-AC HEC regimen received palonosetron (PALO) plus dexamethasone (DEX) to prevent CINV
- Bolus IV push arm: Single dose of PALO 0.25 mg administered IV over 30 seconds + DEX
- IV infusion arm: Single dose of PALO 0.25 mg administered IV over 30 minutes + DEX
- Primary endpoint: Noninferiority of palonosetron IV infusion to bolus IV push
- Complete response: No emesis or rescue medication use during the acute phase (0-24 hours after chemotherapy)
- Prespecified noninferiority margin was set at -15%
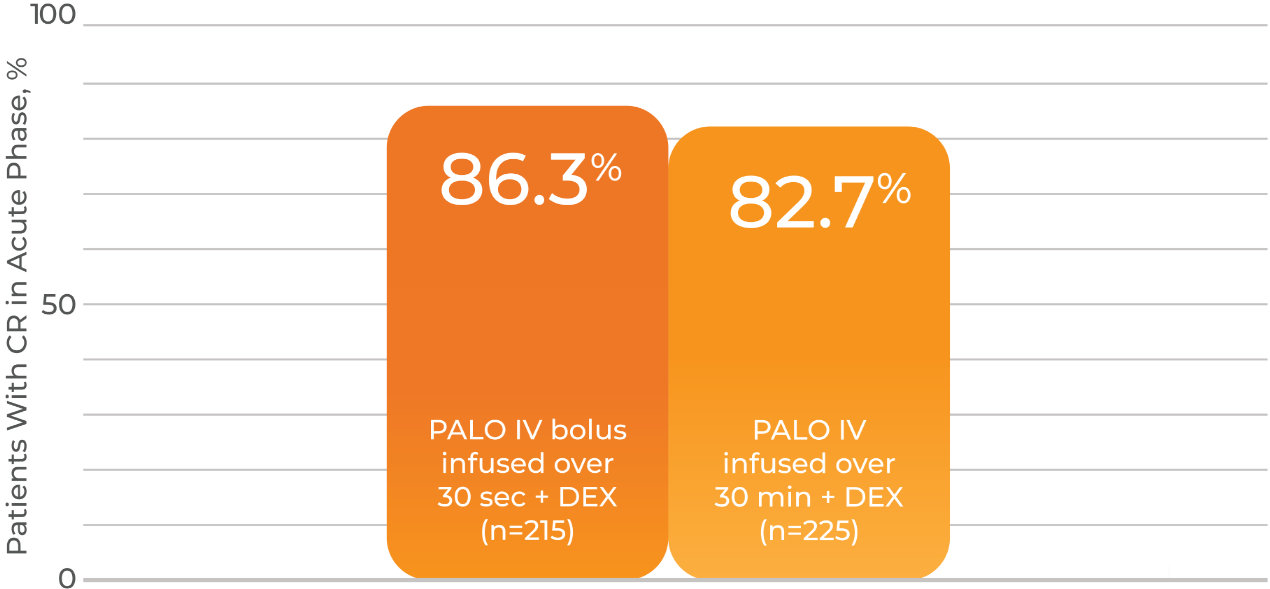
- Patients achieving complete response in the acute phase for IV infusion and IV bolus push were 82.7% and 86.3%, respectively
- Difference of -3.4%; 99% CI -12.0% to 5.2%
- The frequency and severity of all reported TEAEs were similar between treatment arms
Fosnetupitant in AKYNZEO injection demonstrated bioequivalence to netupitant in AKYNZEO capsules1,2
- AKYNZEO injection is the only combination agent that includes a 5-HT3 RA (palonosetron) with an NK-1 RA (netupitant)
- Netupitant, the NK-1 RA in AKYNZEO capsules, is not water soluble, so AKYNZEO injection includes the soluble prodrug, fosnetupitant
- Fosnetupitant is rapidly converted to netupitant after IV administration
Plasma concentrations after single dose of fosnetupitant2
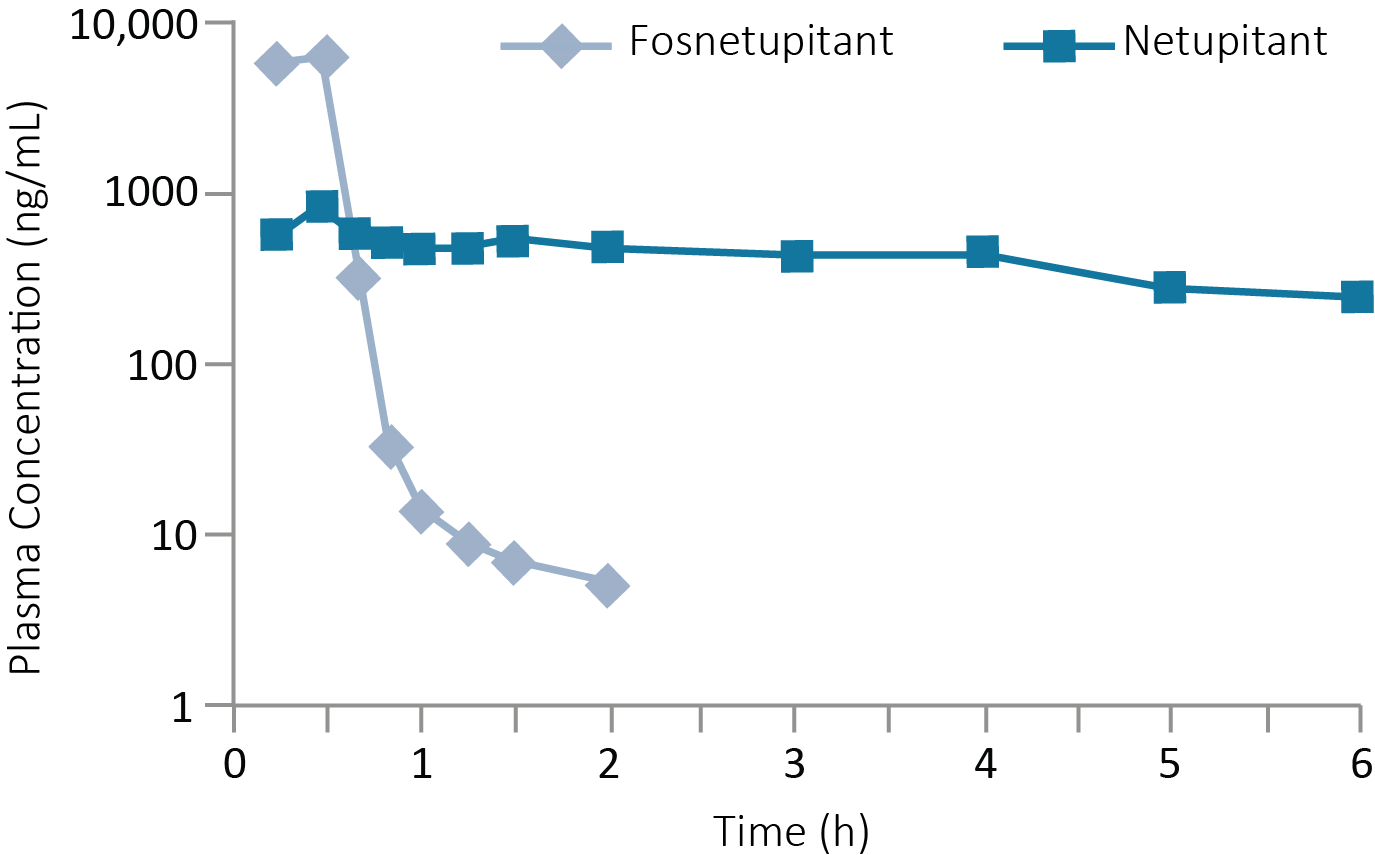
Fosnetupitant plasma concentrations decreased rapidly, while netupitant plasma concentrations remained steady over time2
- The pharmacologic effects of fosnetupitant can be attributed to the activity of netupitant
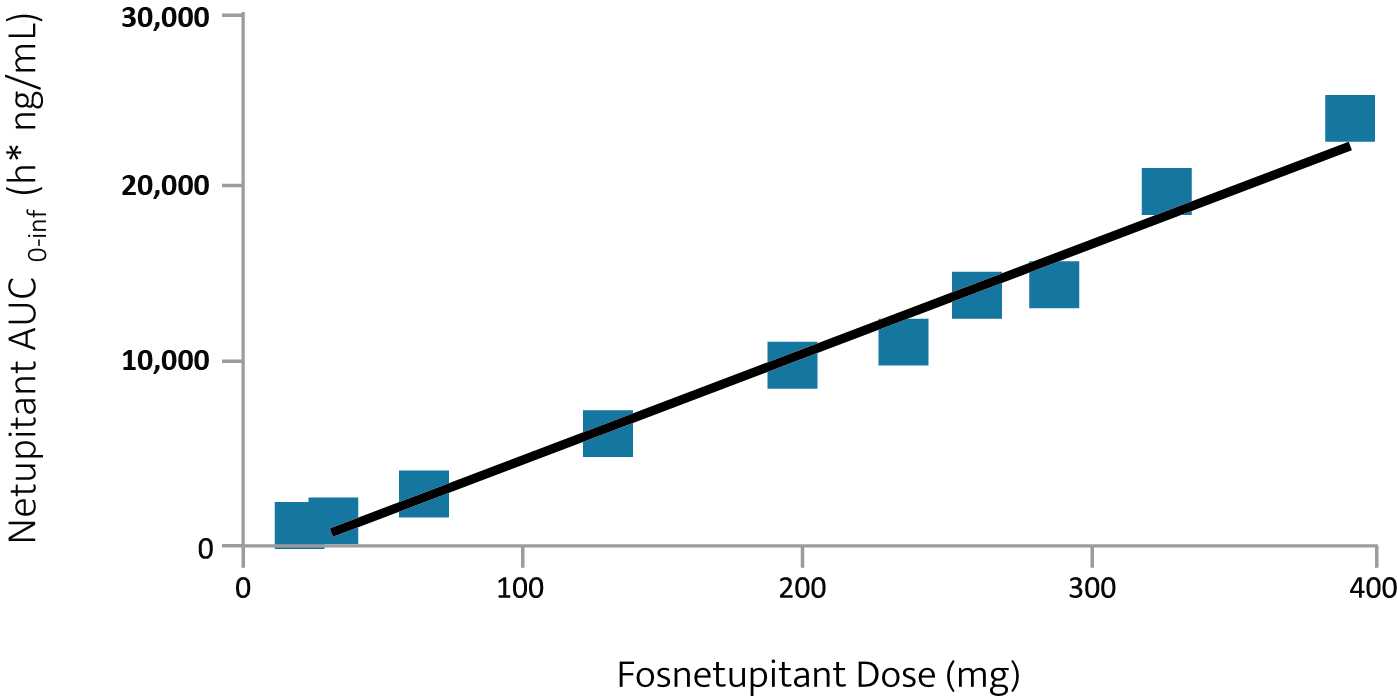
- An IV fosnetupitant dose of 235 mg (260 mg of fosnetupitant chloride hydrochloride) achieved a similar netupitant area under the curve (AUC) as 300 mg oral netupitant
Linear relationship between fosnetupitant dose and netupitant AUC2
The safety profile of AKYNZEO injection was generally similar to AKYNZEO capsules1,3
- Phase III, multinational, randomized, double-blind, multicycle safety study3
- 404 patients receiving a cisplatin-based HEC regimen for solid tumors received either oral or IV AKYNZEO to prevent CINV1,3
- Median age: 60 years; 46% women; 99.5% white; 0.3% Asian; 0.3% Hispanic1
- Oral arm: Single dose of AKYNZEO capsule + DEX (n=201)
- IV arm: Single IV dose of AKYNZEO + DEX (n=203)
- DEX dose was PO 12 mg on Day 1, followed by 8 mg on Days 2-4 for both arms
- Safety profile was generally similar between oral and IV formulations1,3
- TRAEs were observed in 8.9% and 9.5% of patients in IV and PO arms, respectively
- Severe TRAEs were observed in 0.5% and 1.0% of patients in IV and PO arms, respectively
- There were no infusion-site reactions related to AKYNZEO injection3
AKYNZEO injection contains
- No polysorbate 80
(a solubilizing agent that can cause severe nonimmunologic anaphylactoid reactions)1,4 - No preservatives1
- No soy or egg lecithin1
In clinical trials
- No infusion-site reactions related to AKYNZEO3
- No anaphylaxis attributed to fosnetupitant (NK-1 RA) reported3
- No significant effect on QT interval1
Hypersensitivity reactions, including anaphylaxis, have been reported in patients receiving palonosetron, one of the components of AKYNZEO, with or without known hypersensitivity to other 5-HT3 receptor antagonists.
- 5-HT3 RA=5-HT3 receptor antagonist; AC=anthracycline plus cyclophosphamide; CINV=chemotherapy-induced nausea and vomiting; HEC=highly emetogenic chemotherapy; IV=intravenous; NK-1 RA=neurokinin-1 receptor antagonist; PO=oral; TRAE=treatment-related adverse event.
*AKYNZEO injection has not been studied for the prevention of nausea and vomiting associated with AC chemotherapy.
