AKYNZEO® provided superior CINV prevention in AC therapy
Complete response with AKYNZEO capsules + dexamethasone following AC chemotherapy treatment1
Complete response (no emesis and no use of antiemetic rescue medication) for 5 days in AC-treated patients, cycle 11
| AKYNZEO capsules (n=724) |
Oral palonosetron 0.5 mg (n=725) |
P values from Cochran-Mantel-Haenszel test, stratified by age, class, and region |
|
|---|---|---|---|
| Primary endpoint: | |||
| Complete response, delayed phase (25 to 120 hours after AC regimen) | 77% | 70% | P=0.001 |
| Major secondary endpoint: | |||
| Complete response, acute phase (0 to 24 hours after AC regimen) | 88% | 85% | P=0.047 |
| Complete response, overall phase (0 to 120 hours after AC regimen) | 74% | 67% | P=0.001 |
See safety profile in AC-treated patients
Sustained efficacy across multiple cycles in AC-treated patients receiving AKYNZEO capsules1,2
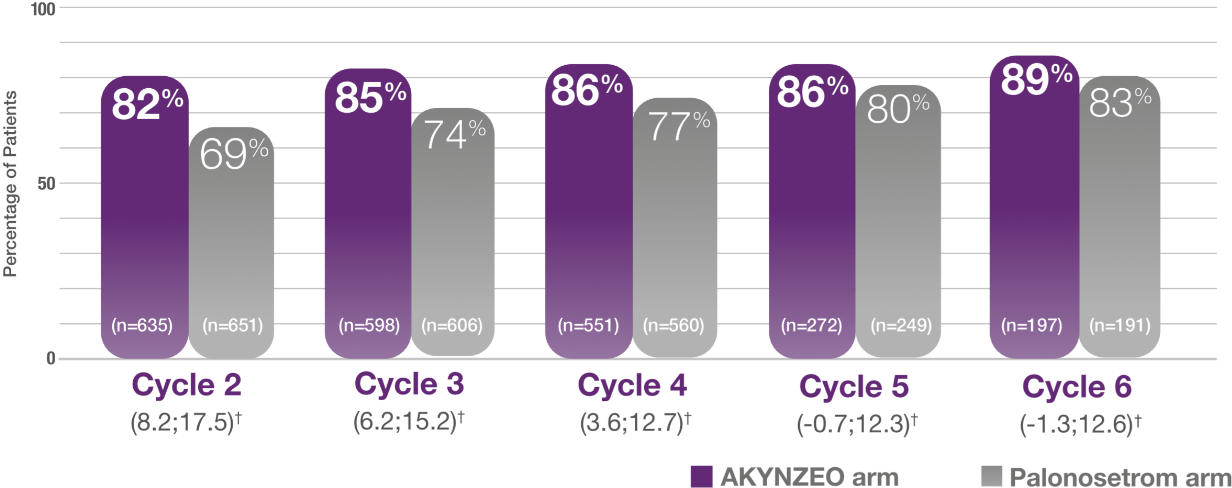
†95% confidence interval.
Complete response in the delayed phase was higher with AKYNZEO among patients receiving AC chemotherapy treatment during all cycles (cycles 2–6)1
After completion of cycle 1, patients had the option to participate in a multiple-cycle extension, receiving the same treatment as assigned in cycle 11,3
- 1438 patients (99%) completed cycle 11
- 1286 patients (88%) continued treatment in the multiple-cycle extension1
AKYNZEO study design in patients receiving AC chemotherapy treatment1
Phase III, multinational, multicenter, randomized, double-blind, double-dummy, parallel-group study evaluating AKYNZEO capsules (n=724) vs oral palonosetron (n=725) in AC-based chemotherapy, which was considered MEC at the time the study was conducted1,3:
- 98% of patients were undergoing chemotherapy for breast cancer3
- Day 1: AKYNZEO capsule + dexamethasone 12 mg vs palonosetron 0.5 mg + dexamethasone 20 mg1
- Days 2-3: No antiemetic treatment1
*AKYNZEO injection has not been studied for the prevention of nausea and vomiting associated with AC chemotherapy.
- AC=anthracycline plus cyclophosphamide; CINV=chemotherapy-induced nausea and vomiting; MEC=moderately emetogenic chemotherapy.
