Clinical data demonstrated the efficacy of AKYNZEO® in preventing CINV
90% of AKYNZEO patients receiving cisplatin had no vomiting and no rescue medication1
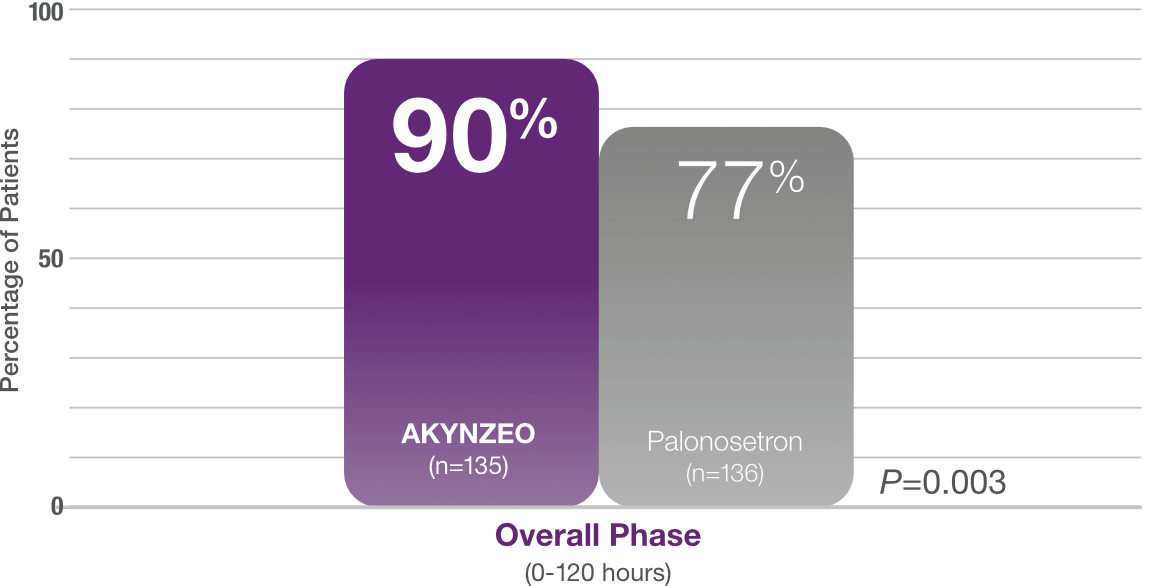
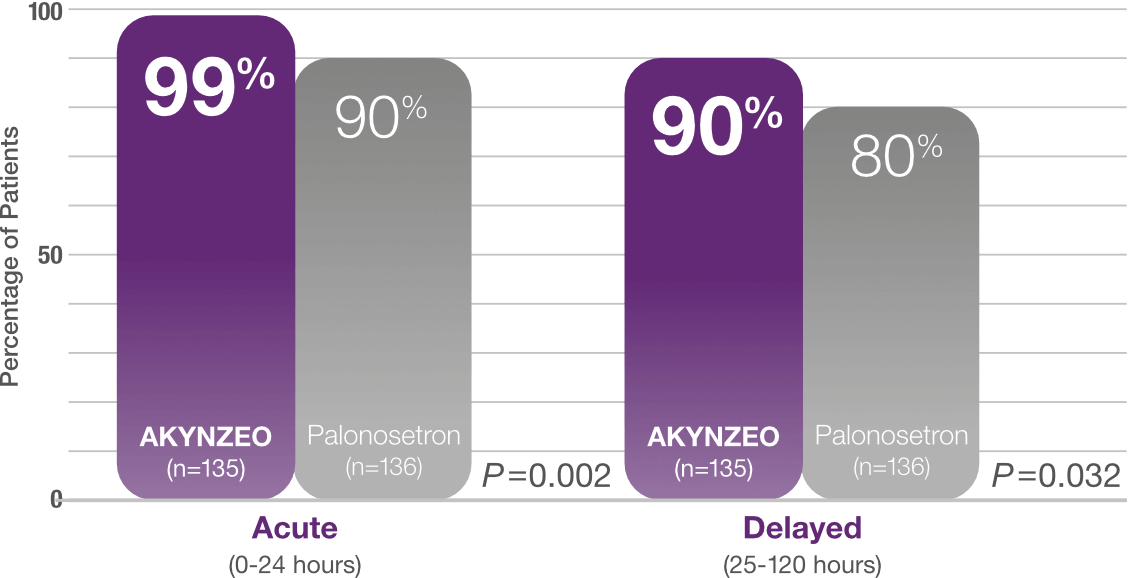
Study design: Multicenter, randomized, parallel, double-blind, controlled trial in chemotherapy-naïve patients (N=694) who received CINV prophylaxis with either a single dose of AKYNZEO capsules + oral dexamethasone 12 mg on Day 1 followed by 8 mg oral dexamethasone once daily on Days 2-4 (n=135) vs a 0.5 mg oral dose of palonosetron + oral dexamethasone 20 mg on Day 1 followed by oral dexamethasone 8 mg twice daily on Days 2-4 (n=136). All patients received cisplatin (median cisplatin dose was 75 mg/m2 for each group) alone or with other chemotherapy agents. The AKYNZEO patients primarily had a diagnosis of lung/respiratory cancer (25.9%), head and neck cancer (24.4%), or ovarian cancer (17.8%). The primary endpoint was complete response in the overall phase (0-120 hours).1,2
AKYNZEO capsules demonstrated superior efficacy to palonosetron1
AKYNZEO injection has established efficacy based on bioequivalence of fosnetupitant to netupitant, and a noninferiority study with palonosetron1,3
≥90% complete response with AKYNZEO in the acute and delayed phase1
AKYNZEO demonstrated significant improvement for secondary endpoints† in acute and delayed phases vs palonosetron, including no emesis, no significant nausea, and complete protection (P≤0.05)2
- AC=anthracycline plus cyclophosphamide; CINV=chemotherapy-induced nausea and vomiting; IV=intravenous; VAS=visual analog scale.
*AKYNZEO injection has not been studied for the prevention of nausea and vomiting associated with AC chemotherapy.
- †Protocol-defined secondary endpoints included rates of no emesis, no significant nausea (VAS <25 mm), and complete protection (complete response + no significant nausea) during acute, delayed, and overall phases.2
